The Daniell cell on the road of electrochemical battery
A type of electrochemical cell consisting of a copper pot filled with a copper(II) sulfate solution, in which an unglazed earthenware container filled with sulfuric acid and a zinc electrode is immersed.

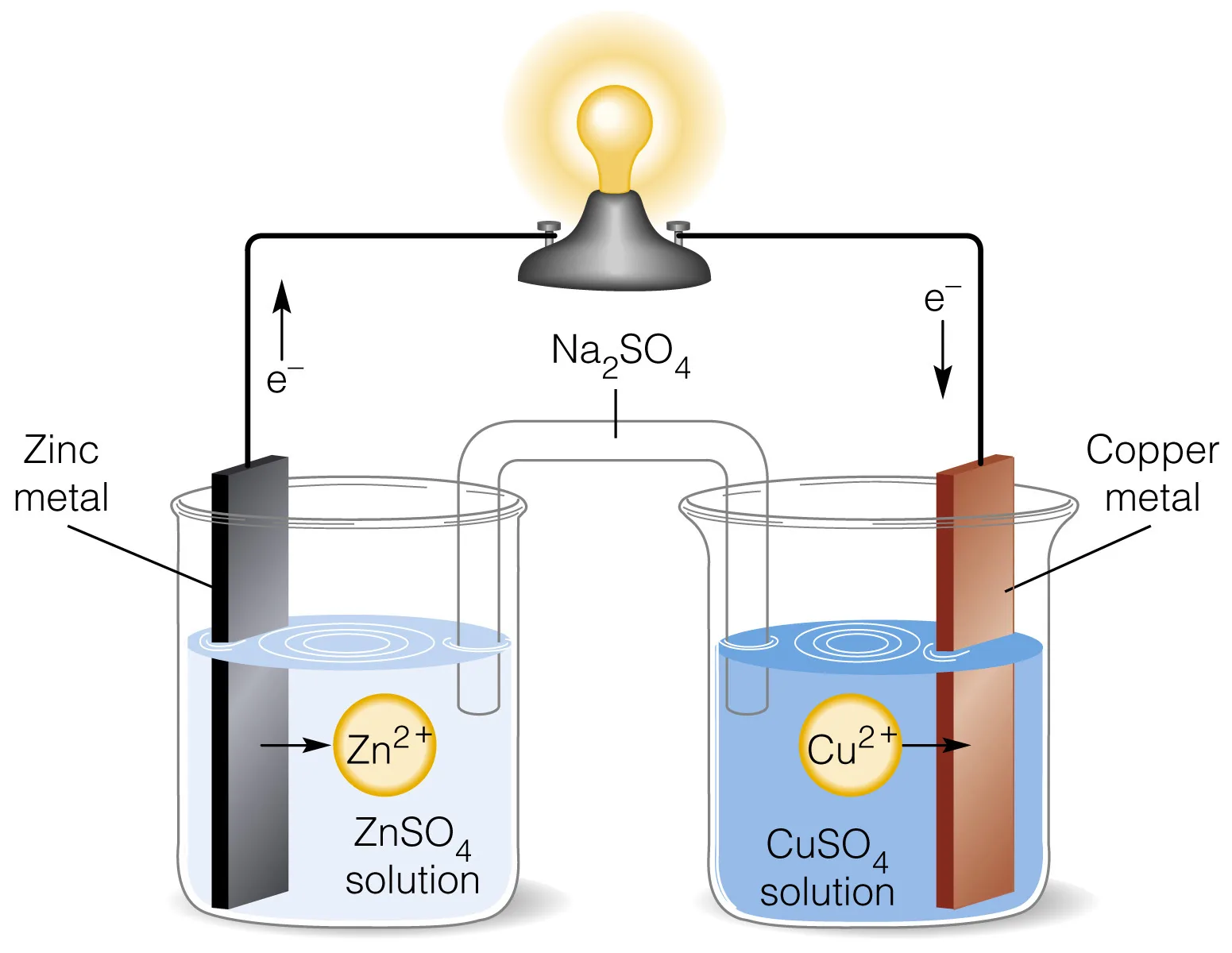
The Daniell cell is a type of electrochemical cell that was invented in 1836 by John Frederic Daniell, a British chemist and meteorologist. It was a significant improvement over the earlier voltaic pile, addressing the issue of hydrogen bubble formation. The Daniell cell consists of a copper pot filled with a copper (II) sulfate solution, in which an unglazed earthenware container filled with sulfuric acid and a zinc electrode is immersed.
Key Points
- The Daniell cell was designed to eliminate the hydrogen bubble problem found in the voltaic pile by using a second electrolyte to consume the hydrogen produced by the first.
- It consists of a copper electrode in a copper sulfate solution (Cu^2+ ions) and a zinc electrode in a sulfuric acid solution (Zn^2+ ions).
- The copper electrode is the positive terminal (cathode), while the zinc electrode is the negative terminal (anode).
- The two half-cells are connected by a salt bridge, which allows the flow of ions to maintain charge balance.
- The Daniell cell operates based on redox reactions, where zinc atoms oxidize at the anode, releasing electrons, and copper ions reduce at the cathode, accepting electrons.
- The overall reaction in the Daniell cell is: Zn(s) + Cu^2+(aq) → Zn^2+(aq) + Cu(s).
- The Daniell cell was a significant advancement in battery technology during its time and found applications in electrical telegraphy.
Usage and Applications
- The Daniell cell can be used to generate electricity or store electricity by consuming an electrode.
- It has been historically used in various applications, including early telegraph systems.
- The Daniell cell is often used in educational settings to demonstrate the principles of electrochemistry and battery operation.
- While the Daniell cell is not commonly used in practical applications today, it played a crucial role in the development of battery technology and understanding of electrochemical processes.
It’s worth mentioning that the Daniell cell is not rechargeable or reversible in its standard configuration. Reversing the current flow by an external source of electromotive force is not possible due to the flow of copper ions into the zinc half-cell, which would result in the discharge of copper ions instead of zinc ions.